What is Senescence?
In 1961, Hayflick and Moorhead conducted various experiments using cell cultures. Cells from human tissues (lung, kidney, skin, muscle, liver, and heart) and fetuses were used to try and cultivate cell lines for long periods of time. The authors found found that cells only replicated a certain number of times before entering into a state of arrest, cellular senescence (Hayflick and Moorehead, 1961). Later on, the term "Hayflick number" was used to describe the number of times a cell replicates before entering into senescence. Hayflick and Moorehead hypothesized that senescence plays an important role in organism aging, as well as cancer prevention (Bennett et al, 2014). Once a cell has entered into senescence, the cell begins to secrete a myriad of factors. The secretion of these factors are known as senescence-associated secretory phenotype (SASP). The SASP has been shown to be associated with inflammation and malignancy (Coppe et al, 2008).
Senescence Overview
Many stresses such as exposure to oxidants, γ-irradiation, UVB light, and DNA damaging chemotherapies, lead to senescence. Overexpression of oncogenes such as Ras and BRAFV600E as well as loss of tumor suppressors like PTEN also enable senescence (Bennett et al, 2014). Perhaps the most common inducer of senescence is telomere erosion. When preparing to divide, a cell replicates its DNA. When DNA is copied, telomere erosion causes the telomere to become shorter. Telomeres are segments of non coding DNA residing on both ends of chromosomes, protecting the coding DNA. When a cell replicates, copying the DNA, telomeres gradually become smaller and smaller until they become so small the coding DNA is in danger. Telomerases are capable of replenishing the shrunken telomeres. Telomerases are enzymes that add telomeric DNA repeats to the end of the telomeres (Bennett et al, 2014). Stem cells, as well as many cancer cells, are where telomerases are found. Cells possessing telomerases have the ability to replicate indefinitely, avoiding cellular senescence. If the telomeres become small enough to potentially harm the coding DNA, then the cell activates a DNA damage response (DDR). A DDR response upregulates inhibitors of cell cycle progression, activating senescence, and halting cell growth (Bennett et al, 2014). A video further explaining the process of telomeres and cellular senescence is presented below.
Developmental Apoptosis and Senescence
Apoptosis has been shown to be very important in development. Limb development relies on apoptosis to help form digits. A study done on regulation of bone morphogenic proteins (BMPs) showed that BMPs mediate the identification of programmed cell death, apoptosis. An experiment done on chickens showed that overexpression of certain dominant negative BMP receptors (dnBMPR-IA or dnBMPR-IB) led to reduced identification of tissues for apoptosis. The result was webbed feet. When apoptotic tissues were identified by applying BMP-2 or BMP-7 "intensified apoptosis" occurred (Guimond et al, 2010).
During development, an abundance of neurons are made to form connections throughout the body. As these neurons search for their target tissue, neurons that do not form connections are degraded through apoptosis. Many other cells, mass produced in the embryo, are eliminated by apoptosis such as mammary tissue in males, suggesting apoptosis regulates patterning in developing embryos. The hypothesized mechanism for this is by altering the cellularity through cell death (Bennett et al, 2014).
Serrano et al, identified senescence in the endolypohatic sac, as well as the mesonephros of mouse and human embryos. Their results led them to the conclusion that senescence has a morphogenetic role analogous to apoptosis. Serrano hypothesized that cellular senescence played a role in tissue patterning. Through magrophate-mediated clearance of senescent cells, or by the overgrowth of nearby cells, causing the cellularity to alter (Storer et al, 2013). Keyes et al, found evidence of senescence in the apical ectodermal ridge and the neural floorplate. The SASP of these cells were hypothesized to induced tissue remodeling (Bennett et al, 2014).
Adult Apoptosis and Senescence
Transient senescent cells in the embryo and the adult are known as "acute senescent cells". Acute senescent cells serve a similar transient purpose to the cells in the embryo, meaning the SASP directs tissue repair and regeneration. Like senescence in development, immunosurveillance clears these cells after their programmed function is performed. Although similar to development, the pathways in which senescence works is different in adults (Bennett et al, 2014).
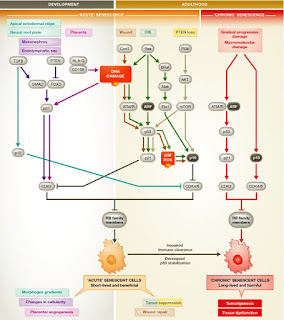
In the elderly, senescent cells may begin to accumulate due to factors like declining immune function, decreased ability to stabilize the master regulator p53 to levels required for apoptosis, or the slow accumulation of macromolecular damage that does not reach the threshold for cell death (Bennett et al, 2014). Distinct differences between apoptosis and senescence in adults will be discussed below in greater detail. Figure 1 depicts a few similarities as well as many differences between senescence pathways during development and adulthood.
Figure 1. (Bennett et al, 2014) Senescence in development and adulthood. This picture shows the differences in pathways from development and adulthood. Following the sequence in the picture lets us see how acute senescent cells, as well as chronic senescent cells are formed in tissues. The color of the line corresponds to location and situation in which senescence occurs, as stated in the picture (Brown line represents wound and wound repair).
Stress
In a few instances, apoptosis is a response to overwhelming stress. Senescence, on the other hand, is a consequence of less severe stress damage (Figure 2). An example of this is a dose dependent response meaning the greater the dose, the more likely apoptosis is induced. A study done using MCF7 breast cancer cells showed this type of dose dependent response. When administered at low levels, Doxorubicin leads to senescence, but at high doses, causes apoptosis (Bennett et al, 2014). Oxidative damage also induces a similar dose dependent effect. F65 and IMr90 human diploid fibroblasts were treated with H2O2. Apoptosis was the result of H2O2 in high doses, while low doses of H2O2 caused senescence to take place (Bennett et al, 2014). Other DNA damaging agents are not dose dependent and elicit a standard response. Basulfan is an example of this. Basulfan gives rise to senescence no matter the concentration (Bennett et al, 2014).
Figure 2. (Bennett et al, 2014). Signaling pathways enforce the choice between cell cycle arrest, senescence, and apoptosis. Depiction of the pathways taken by a cell that lead to senescence or apoptosis are shown. The level of cellular stress plays an important role on which pathway is taken. The color of the lines indicate the level of cellular stress that induced it (low levels of stress are gray)
Pathways
The balance between pro-senescent and pro-apoptotic pathways, play a crucial role in deciding if a cell should undergo apoptosis or senescence. A pathway controlled by p53, a tumor suppressor gene, is one such pathway. The pathway regulating p53, is shown to be involved in both apoptosis as well as senescence. MEF cells expressing the hypomorphic R172P p53 mutation senesce instead of undergoing apoptosis in response to UVB. The cells do not up regulate the pro-apoptotic factors PUMA or NOXA, and they express high levels of BCL, a pro-survival gene (Bennett et al, 2014). On the other hand, human diploid fibroblasts, treated with H2O2 doses sufficient to induce a mixture of apoptosis and senescence, lead to p53 induction. In cells undergoing apoptosis, the levels of p53 were twice as high as the cells that underwent senescence (Bennett et al, 2014).
Resistance or Sensitivity
If apoptosis is believed to be an alternative fate to senescence for a cell, then a hypothesis would claim that pro-senescent cellular changes are anti-apoptotic. If pro-senescent changes are anti-apoptotic, then senescent cells should be resistant to apoptosis (Bennett et al, 2014). Seluanov et al, provided evidence for the existence of apoptosis resistance in replicatively senescent HDF cells. Seluanov showed this occurs through p53 signaling (Figure 3). He found early-passage WI-38 cells undergo p53-dependent apoptosis in response to actinomycin D, low-dose cisplatin, or UVB irradiation, whereas p53-independent apoptosis occurred with high-dose cisplatin and etopside. When the senescent cells were hit with p53-dependent apoptotic stimuli, then they underwent necrosis instead. The exogenous expression of p53 in the senescent cells restored their p53-dependent apoptosis function (Bennett et al, 2014). Another outcome of apoptosis resistance can also be cell survival. Senescence activated by mild H2O2 promotes survuval rather than apoptosis when in response to apoptotic stimuli such as UVB or high dosage H2O2 (Bennett et al, 2014). Also, replicative senescent human fibroblasts resistant to apoptosis in response to serum, withdrawal by maintaining high levels of BCL-2 (Bennett et al, 2014).
Figure 3. (Bennett et al, 2014) The interplay between senescence and apoptosis is cell type specific. The pathways in which apoptosis sensitivity or resistance is shown. The color of the line depicts cause of stress and the end fate for the cell.
Signaling Within Tissues
The pro-inflammatory secretion
of growth factors and cytokines from senescent cells (SASP) has the potential to
generate prolonged paracrine signaling (cell communication with nearby cells inducing a change in the cells). Through this, apoptosis is a natural or intrinsic mechanism in the cell, compared to the dual cell autonomous
and non-autonomous nature of senescent cells (Bennett et al, 2014). Emerging data suggests the
presence of senescent cells have an advantage over apoptosis due to this ability
to communicate with other cells. From this data, signaling from senescent cells within tissues can possibly be both beneficial and detrimental (Figure 4). Various
components of the SASP, promote pre-malignant cell growth, or invasion, through
their ability to induce angiogenesis, epithelial–mesenchymal transitions, and
differentiation within the local microenvironment. The results are clearly
pro-neoplastic and thus are detrimental side effects of the SASP (Bennett et al, 2014).
Several studies
have suggested the SASP is not always pro-tumorigenic. First, the SASP can
reinforce and maintain the senescent state in cell culture models of senescence. Next, the SASP attracts the immune system, clearing out both premalignant and
established tumor cells by phagocytosis or cytotoxic-mediated killing, through
a “senescence surveillance” process. Senescence surveillance entails both innate and adaptive
immune responses (Bennett et al, 2014). Oncogene-induced, pre-malignant hepatocytes present many
features of senescent cells, including high levels of p16Ink4a, p21 and senescence-associated (SA)-β–galactosidase
activity. The SASP generated by senescent cells are believed to initiate a CD4+-T-cell-mediated adaptive immune response, subsequently
removing these pre-malignant lesions (Bennett et al, 2014). Reactivation of p53 in a
Ras-induced liver-carcinoma mouse model resulted in rapid regression of the
existing tumor. Surprisingly, the tumors were not eliminated through apoptosis, but through cellular senescence and SASP, keeping consistent with observations from the sarcoma mouse model. The SASP generated within liver tumors
triggers the innate immune system to respond to the senescent cells, removing them through the action of macrophages, neutrophils, and NK cells (Bennett et al, 2014).
Figure 4. (Bennett et al, 2014) Consequences of senescence and apoptosis in stressed tissues. The consequences of apoptosis and senescence are illustrated in this figure. Oncogenic insults and tissue damage are displayed on the top and bottom respectively. The outcome for the cell, as well as the surrounding cells, due to senescence and apoptosis are shown.



No comments:
Post a Comment